She still has moderate to severe endometriosis pain despite treatment. Is it time for a change?
Unresolved pain
Patients with unresolved pain tend to cycle through medical treatment2,3
Patients treated with contraceptives or NSAIDs can still have unresolved pain2
Is it time for another treatment option?
In a survey of 931 women, about 70% who were treated for endometriosis still had pain3*
More than 1-2 office visits a year for her unresolved endometriosis pain suggests she’s ready for a different therapy4
~46% of women cycle through ≥3 medical treatments2†
Women with partial relief from existing therapies may benefit from an alternate approach
Beyond surgery
Women often deal with multiple surgeries and incomplete long-term relief3,5
Even with surgery, patients can still have recurrent pain5
ACOG guidelines recommend both medical and surgical treatments as effective options for endometriosis pain6

According to ACOG, up to 80% of women experience recurrent endometriosis-associated pain within 2 years of surgery5
In a survey that included 931 women with a laparoscopic and/or histological diagnosis of endometriosis: Women frequently underwent >1 surgery (mean=2.2 surgeries)3
Women with unresolved or recurrent endometriosis pain post surgery may benefit
from an alternate approach to help achieve pain relief
Impact of endometriosis pain
Endometriosis is a chronic, progressive disease and patients in debilitating pain should be treated with urgency6-9
Ongoing, unresolved endometriosis pain early in the disease journey may have later consequences8,10
Endometriosis-associated pain may significantly impact the day-to-day activities of women's lives personally and professionally. These include3,11:
1Dysmenorrhea
2Non-menstrual pelvic pain
3Dyspareunia
Consider an alternate approach earlier for the pain reduction she needs
Estrogen suppression
Suppressing estrogen is 1 way to effectively manage the pain of endometriosis, an estrogen-driven disease12
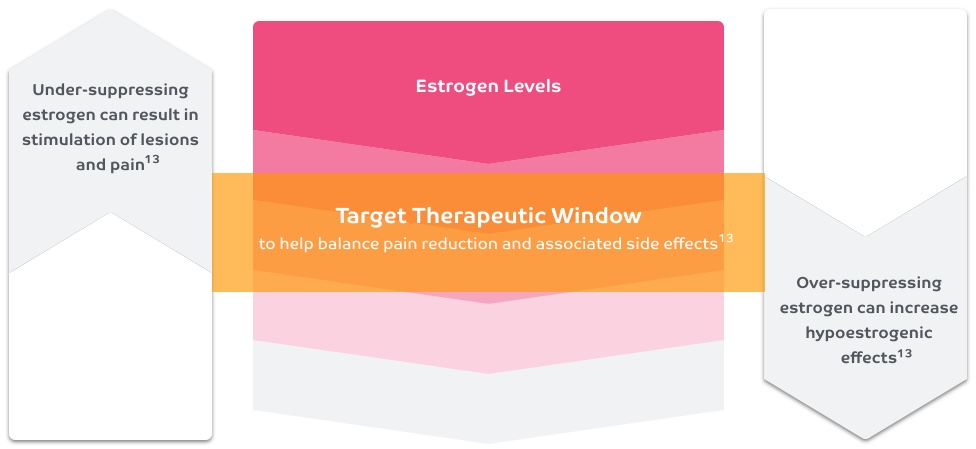


Consider the balance between pain relief and side effects when suppressing estrogen
*Based on a cross-sectional, questionnaire-based survey among women with laparoscopically and/or histographically diagnosed endometriosis treated in 12 tertiary care centers in 10 countries who had at least one contact related to endometriosis-associated symptoms during 2008 with a participating center.
†From a survey about the lifetime treatment experiences of 1160 women with surgically diagnosed endometriosis. The objective of this study was to report on the lifetime therapeutic practices and effectiveness of medical, surgical, and alternative treatments for endometriosis from the patients' perspective.
Indication and Important Safety Information
INDICATION1
ORILISSA® (elagolix) is indicated for the management of moderate to severe pain associated with endometriosis.
IMPORTANT SAFETY INFORMATION1
CONTRAINDICATIONS
- ORILISSA is contraindicated in women who are pregnant (exposure to ORILISSA early in pregnancy may increase the risk of early pregnancy loss), in women with known osteoporosis or severe hepatic impairment, or with concomitant use of strong organic anion transporting polypeptide (OATP) 1B1 inhibitors (e.g., cyclosporine and gemfibrozil).
WARNINGS AND PRECAUTIONS
Bone Loss
- ORILISSA causes a dose-dependent decrease in bone mineral density (BMD), which is greater with increasing duration of use and may not be completely reversible after stopping treatment.
- The impact of ORILISSA-associated decreases in BMD on long-term bone health and future fracture risk is unknown. Consider assessment of BMD in patients with a history of low-trauma fracture or other risk factors for osteoporosis or bone loss, and do not use in women with known osteoporosis.
- Limit the duration of use to reduce the extent of bone loss.
Change in Menstrual Bleeding Pattern and Reduced Ability to Recognize Pregnancy
- Women who take ORILISSA may experience a reduction in the amount, intensity, or duration of menstrual bleeding, which may reduce the ability to recognize the occurrence of pregnancy in a timely manner. Perform pregnancy testing if pregnancy is suspected, and discontinue ORILISSA if pregnancy is confirmed.
Suicidal Ideation, Suicidal Behavior, and Exacerbation of Mood Disorders
- Suicidal ideation and behavior, including one completed suicide, occurred in subjects treated with ORILISSA in the endometriosis clinical trials.
- ORILISSA users had a higher incidence of depression and mood changes compared to placebo and ORILISSA users with a history of suicidality or depression had an increased incidence of depression. Promptly evaluate patients with depressive symptoms to determine whether the risks of continued therapy outweigh the benefits. Patients with new or worsening depression, anxiety, or other mood changes should be referred to a mental health professional, as appropriate.
- Advise patients to seek immediate medical attention for suicidal ideation and behavior. Reevaluate the benefits and risks of continuing ORILISSA if such events occur.
Hepatic Transaminase Elevations
- In clinical trials, dose-dependent elevations of serum alanine aminotransferase (ALT) at least 3 times the upper limit of the reference range occurred with ORILISSA.
- Use the lowest effective dose and instruct patients to promptly seek medical attention in case of symptoms or signs that may reflect liver injury, such as jaundice.
- Promptly evaluate patients with elevations in liver tests to determine whether the benefits of continued therapy outweigh the risks.
Reduced Efficacy with Estrogen-Containing Contraceptives
- Based on the mechanism of action of ORILISSA, estrogen-containing contraceptives are expected to reduce the efficacy of ORILISSA. The effect of progestin-only contraceptives on the efficacy of ORILISSA is unknown.
- Advise women to use non-hormonal contraceptives during treatment and for one week after discontinuing ORILISSA.
ADVERSE REACTIONS
- The most common adverse reactions (>5%) in clinical trials included hot flushes and night sweats, headache, nausea, insomnia, amenorrhea, anxiety, arthralgia, depression-related adverse reactions, and mood changes.
These are not all the possible side effects of ORILISSA.
Safety and effectiveness of ORILISSA in patients less than 18 years of age have not been established.
US-ORIL-200330
For more information, please click here for full Prescribing Information.
References:
1. ORILISSA [package insert]. North Chicago, IL: AbbVie Inc. 2. Sinaii N, Cleary SD, Younes N, Ballweg ML, Stratton P. Treatment utilization for endometriosis symptoms: a cross-sectional survey study of lifetime experience. Fertil Steril. 2007;87(6):1277-1286. 3. De Graaff AA, D'Hooghe TM, Dunselman GAJ, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685. 4. Data on file. In Play Analytics; May 2020. 5. American College of Obstetricians and Gynecologists. Frequently asked questions. FAQ013. Gynecologic problems. https://www.acog.org/patient-resources/faqs/gynecologic-problems/endometriosis. Updated October 2012. Accessed January 8, 2020. 6. American College of Obstetricians and Gynecologists. Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223-236. 7. Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion [published correction appears in Fertil Steril. 2015;104(2):498]. Fertil Steril. 2014;101(4):927-935. 8. Agarwal SK, Chapron C, Giudice LC, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220(4):354.e1-354.e12. 9. Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Ann Rev Physiol. 2011;73:163-182. 10. Casper RF. Introduction: a focus on the medical management of endometriosis. Fertil Steril. 2017;107(3):521-522. 11. Fourquet J, Gao X, Zavala D, et al. Patients' report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424-2428. 12. Fischer JR. APGO Educational Series on Women's Health Issues. Diagnosis & management of endometriosis: pathophysiology to practice. Association of Professors of Gynecology and Obstetrics; 2012. 13. Barbieri RL. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J Reprod Med. 1998;43(suppl 3):287-292.







