ORILISSA patient counseling aid
What is ORILISSA?
ORILISSA is a pill that's clinically proven to help relieve moderate to severe endometriosis pain. It's not a painkiller, injection, surgery, or birth control and it does not contain hormones.
How does ORILISSA work?
- ORILISSA works in its own way. It's a pill that dials down estrogen. Lower estrogen levels can help manage endometriosis pain
- ORILISSA is available in 2 doses. Each dose lowers estrogen by different amounts so you and your gynecologist can choose which dose is best for your individual needs
What were the clinical trial results?
ORILISSA was proven to reduce 3 common types of endometriosis pain*:
1 Painful periods
2 Pelvic pain in between periods
3 Pain with sex
*There are 2 different doses of ORILISSA: 150 mg (taken once a day) or 200 mg (taken twice a day). Only the 200 mg dose was proven to work for pain with sex.
It was studied in the largest endometriosis clinical trial program to date
The program, made up of two 6-month clinical studies, showed that ORILISSA reduces endometriosis pain. It studied the 2 doses of ORILISSA (150 mg and 200 mg) compared to placebo in:

When can I expect to feel relief with ORILISSA?
ORILISSA starts working in the body to lower estrogen after about 24 hours. However, pain relief will take longer. In the clinical studies, ORILISSA was proven to provide pain relief at 3 months.
What are the common side effects?
Most common side effects reported by women in the clinical trials:
The percentage of women who experienced these side effects is shown below.
ORILISSA 150 mg once daily*
ORILISSA 200 mg twice daily†
Placebo (a pill with no active medicine)‡
Hot flash and night sweats
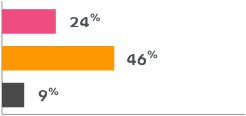
Headache

Nausea

Insomnia (trouble sleeping)

Mood altered, mood swings

Amenorrhea
(absence of periods)

Depressed mood, depression, depressive symptoms, and/or tearfulness

Anxiety

Arthralgia (joint pain)

*Percentage based on 475 women taking ORILISSA 150 mg in 2 studies.
†Percentage based on 477 women taking ORILISSA 200 mg in 2 studies.
‡Percentage based on 734 women taking placebo in 2 studies.
These are not all the possible side effects of ORILISSA. For more information, ask your doctor or pharmacist. Always tell your doctor if you have any side effect that bothers you or does not go away.
How do you take ORILISSA?
ORILISSA is a daily pill available in 2 doses:
ORILISSA 150 mg
One pill taken once a day, at about the same time each day, with or without food

OR


ORILISSA 200 mg
One pill taken twice a day, for a total of 400 mg a day, at about the same time each day, with or without food


AND
Your gynecologist will recommend the lowest effective dose based on your symptoms and treatment goals. Both the pain relief and any side effects you may experience could be different, depending on the dose you take and how your body responds.
Because ORILISSA works throughout your menstrual cycle, it’s important to take ORILISSA every day, exactly as your gynecologist prescribed—even if you’re feeling better. ORILISSA is not a medication you take only as needed.
It does not contain hormones and does not prevent pregnancy.
Starting ORILISSA
Once you and your doctor have decided ORILISSA is right for you, be sure to:
- Review the Important Safety Information and additional considerations below
- Verify you are not pregnant or start within 7 days after you start your period
- Discuss an effective method of nonhormonal birth control to use during your treatment with ORILISSA and for 28 days after you stop treatment
- Birth control pills that contain estrogen may make ORILISSA less effective. It’s not known how well ORILISSA will work while you’re taking progestin-only birth control
- Discontinue ORILISSA if pregnancy occurs. There is a pregnancy registry that monitors outcomes in women who become pregnant while treated with ORILISSA. Enroll by calling 1-833-782-7241
- Schedule a follow-up appointment
Indication and Important Safety Information
INDICATION1
ORILISSA® (elagolix) is indicated for the management of moderate to severe pain associated with endometriosis.
IMPORTANT SAFETY INFORMATION1
CONTRAINDICATIONS
- ORILISSA is contraindicated in women who are pregnant (exposure to ORILISSA early in pregnancy may increase the risk of early pregnancy loss), in women with known osteoporosis or severe hepatic impairment, or with concomitant use of strong organic anion transporting polypeptide (OATP) 1B1 inhibitors (e.g., cyclosporine and gemfibrozil).
WARNINGS AND PRECAUTIONS
Bone Loss
- ORILISSA causes a dose-dependent decrease in bone mineral density (BMD), which is greater with increasing duration of use and may not be completely reversible after stopping treatment.
- The impact of ORILISSA-associated decreases in BMD on long-term bone health and future fracture risk is unknown. Consider assessment of BMD in patients with a history of low-trauma fracture or other risk factors for osteoporosis or bone loss, and do not use in women with known osteoporosis.
- Limit the duration of use to reduce the extent of bone loss.
Change in Menstrual Bleeding Pattern and Reduced Ability to Recognize Pregnancy
- Women who take ORILISSA may experience a reduction in the amount, intensity, or duration of menstrual bleeding, which may reduce the ability to recognize the occurrence of pregnancy in a timely manner. Perform pregnancy testing if pregnancy is suspected, and discontinue ORILISSA if pregnancy is confirmed.
Suicidal Ideation, Suicidal Behavior, and Exacerbation of Mood Disorders
- Suicidal ideation and behavior, including one completed suicide, occurred in subjects treated with ORILISSA in the endometriosis clinical trials.
- ORILISSA users had a higher incidence of depression and mood changes compared to placebo and ORILISSA users with a history of suicidality or depression had an increased incidence of depression. Promptly evaluate patients with depressive symptoms to determine whether the risks of continued therapy outweigh the benefits. Patients with new or worsening depression, anxiety, or other mood changes should be referred to a mental health professional, as appropriate.
- Advise patients to seek immediate medical attention for suicidal ideation and behavior. Reevaluate the benefits and risks of continuing ORILISSA if such events occur.
Hepatic Transaminase Elevations
- In clinical trials, dose-dependent elevations of serum alanine aminotransferase (ALT) at least 3 times the upper limit of the reference range occurred with ORILISSA.
- Use the lowest effective dose and instruct patients to promptly seek medical attention in case of symptoms or signs that may reflect liver injury, such as jaundice.
- Promptly evaluate patients with elevations in liver tests to determine whether the benefits of continued therapy outweigh the risks.
Reduced Efficacy with Estrogen-Containing Contraceptives
- Based on the mechanism of action of ORILISSA, estrogen-containing contraceptives are expected to reduce the efficacy of ORILISSA. The effect of progestin-only contraceptives on the efficacy of ORILISSA is unknown.
- Advise women to use non-hormonal contraceptives during treatment and for one week after discontinuing ORILISSA.
ADVERSE REACTIONS
- The most common adverse reactions (>5%) in clinical trials included hot flushes and night sweats, headache, nausea, insomnia, amenorrhea, anxiety, arthralgia, depression-related adverse reactions, and mood changes.
These are not all the possible side effects of ORILISSA.
Safety and effectiveness of ORILISSA in patients less than 18 years of age have not been established.
US-ORIL-200330
For more information, please click here for full Prescribing Information.




